Fine lines are easy to spot, but what’s happening underneath them?
Why Skin Aging Is More Than Surface-Level
What we see on the surface—wrinkles, dullness, slackening—is only the final chapter of a complex cellular story. Skin aging involves a cascade of physiological changes, many of which occur silently for years before showing up in the mirror. From DNA damage to mitochondrial decline, from hormonal shifts to impaired barrier repair, the process is both internal and external.
This article draws directly from peer-reviewed dermatology research and long-term ageing studies. It’s a clear explanation of how skin changes over time—and what drives those changes beneath the surface. It’s a map of how skin ages—and why.

Skin aging isn’t a single process but an accumulation of molecular stressors and systemic shifts.
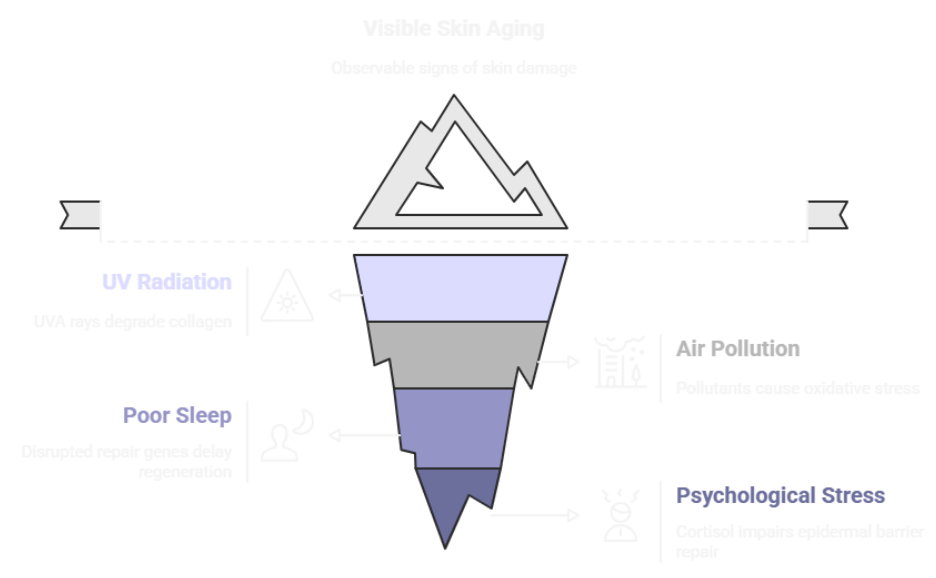
How you live and what you’re exposed to can change how your skin ages.
Internal and External Skin Aging: Two Parallel Pathways
Intrinsic (Chronological) Aging
Intrinsic aging is largely influenced by genetics and biological programming. It occurs in all skin, regardless of sun exposure, and becomes increasingly apparent with age. Between the ages of 30 and 80, the epidermis can thin by up to 50%. There’s a marked flattening of the dermoepidermal junction, with around a 35% reduction in surface area, which weakens nutrient and signal exchange between the dermis and epidermis.
Other internal changes include reduced fibroblast activity, decreased production of collagen types I and III, lower integrin expression, and mild reductions in skin hydration. Skin becomes finer in texture, less elastic, and gradually more fragile.
Extrinsic Aging
In contrast, extrinsic aging is driven by external aggressors. Ultraviolet radiation is the most potent factor, but it’s not the only one. Air pollution, poor nutrition, tobacco smoke, sleep disruption, and chronic psychological stress all contribute.
Unlike intrinsic aging, which results in thinning, extrinsically aged skin often shows thickening of the outermost layer (the stratum corneum), primarily due to impaired cell turnover and chronic inflammation. Sun-damaged skin also displays reduced expression of type VII collagen, fragmentation of fibrillin fibers, and accumulation of dystrophic elastic fibers—a condition known as solar elastosis. These alterations collectively undermine skin structure, leading to deeper wrinkles, irregular pigmentation, and visible coarseness.
Clinical Comparison
In sun-protected areas, skin typically appears pale, finely wrinkled, and dry. In contrast, photoaged areas show deeper wrinkles, visible sagging, rough texture, and discoloration. These aren’t just aesthetic differences—they reflect measurable changes in tissue architecture.
Clinical Point: Intrinsically aged skin reflects genetic tempo. Extrinsically aged skin reflects environmental impact layered onto that baseline.

The Skin Exposome: Environmental Influences on Aging
The term “skin exposome” refers to the full spectrum of external and lifestyle-related factors that influence skin function and accelerate visible aging. First defined in systems biology, this concept is now essential in dermatology because it contextualizes why skin ages differently in different environments—even in genetically similar individuals.
The most influential exposome elements include:
- UV radiation, especially UVA, which penetrates deeply and stimulates matrix metalloproteinases that degrade collagen.
- Airborne pollutants, such as nitrogen dioxide and particulate matter, which trigger oxidative stress and chronic inflammation.
- Poor sleep, which disrupts circadian repair genes like BMAL1, delaying nighttime skin regeneration.
- Psychological stress, which increases cortisol and impairs epidermal barrier repair.
These exposures can interact. For example, UV and pollution together can significantly deplete the skin’s antioxidant capacity, increasing susceptibility to inflammation and barrier dysfunction.
Chronological Aging vs. Photoaging: Molecular Differences That Matter
While both forms of aging ultimately lead to dermal thinning and structural weakening, their mechanisms differ.
Chronological aging is associated with reduced mitotic activity, slower epidermal turnover, and diminished synthesis of extracellular matrix components. Mitochondrial DNA mutations accumulate, telomeres shorten, and DNA repair mechanisms decline. These changes unfold predictably over time.
Photoaging, on the other hand, involves direct DNA damage from UV radiation, increased expression of MMPs that degrade collagen, and the deposition of abnormal elastic material in the dermis. Notably, the architecture of photoaged skin often resembles that of a poorly healed wound. There is persistent low-grade inflammation, impaired cellular adhesion, and a thickened, dysfunctional stratum corneum (Makrantonaki & Zouboulis, 2007).

Intrinsic aging is passive and consistent.
Photoaging is active and inflammatory.
Treating both requires different strategies—even if the end result appears similar on the surface.
Key Molecular Mechanisms of Skin Aging
Skin aging isn’t a result of one thing going wrong—it’s the culmination of intersecting molecular shifts that gradually weaken structural integrity and biological function. We know this from studies of photoaged skin, intrinsically aged skin, and genetic conditions that accelerate aging. What’s critical is not just identifying these mechanisms, but understanding how they interact.
DNA Damage and Repair Decline
With age, cells become less efficient at repairing DNA. This includes both nuclear DNA and mitochondrial DNA (mtDNA). Ultraviolet (UV) radiation increases DNA fragmentation, while intracellular repair enzymes decline in function. Over time, this leads to an accumulation of errors, triggering senescence or apoptosis. Mitochondrial mutations in aged skin impair ATP production, which means cells lose energy needed for regeneration and defense. Studies have shown that telomere shortening and declining telomerase activity further reduce cellular lifespan.
Oxidative Stress and Reactive Oxygen Species (ROS)
Skin is constantly exposed to oxidative stress from both internal metabolism and external sources. ROS—highly reactive oxygen-based molecules—attack cell membranes, proteins, and DNA. In youth, skin has sufficient antioxidant capacity to neutralize them. But with age, these defenses weaken. UV radiation and pollution amplify ROS levels, leading to damage that accumulates faster than it can be repaired. The connection between oxidative stress and aging has been well-documented across multiple tissues, not just skin.
Mitochondrial Decline
Mitochondria are the main producers of cellular energy, and their function deteriorates with age. This isn’t a side note; it’s central to how skin loses vitality. When mitochondria falter, cells produce less ATP and more ROS, initiating a feedback loop of stress and energy deficiency. This accelerates cell death and reduces skin’s ability to repair itself.
Matrix Breakdown via MMPs
One of the most visible consequences of aging is collagen loss. A major contributor is the increase in matrix metalloproteinases (MMPs), enzymes that break down extracellular matrix proteins like collagen and elastin. MMP-1 specifically degrades type I collagen, while MMP-9 targets elastin and fibrillin. Their expression is induced by UV exposure, and the resulting imbalance between collagen breakdown and synthesis is a hallmark of photoaged skin. This breakdown is well-documented histologically as solar elastosis and fibrillar fragmentation.

Pro tip: Ingredients like stabilized vitamin C or retinoids can help modulate MMP activity. But these need to be formulated for skin stability, or they risk oxidation and loss of function.
Hormonal Shifts
Estrogen plays a key role in maintaining skin thickness, hydration, and elasticity. As estrogen levels decline during menopause, these structural qualities diminish. The presence of estrogen receptors in both the dermis and epidermis supports this link. Hormone replacement therapy (HRT) has shown benefits in improving collagen density and hydration in some studies, though its use must be weighed against systemic risks .
Structural and Functional Consequences in Skin
Epidermal Layer
In intrinsic aging, the epidermis thins and cellular turnover slows. Integrin expression declines, affecting how keratinocytes anchor to the basement membrane. In contrast, photoaged skin often shows a thickened stratum corneum due to impaired desquamation and prolonged inflammation. This results in dullness, uneven texture, and compromised barrier function.
Dermal Architecture
Collagen production decreases while degradation rises, especially in photoexposed areas. Elastic fibers become fragmented, and their remnants accumulate in clumps—a process called solar elastosis. Glycosaminoglycans like versican increase but often bind to damaged structures, rendering them less functional. Together, these changes create skin that is less resilient, less firm, and visibly aged.
Vascular and Immune Alterations
Capillaries in aged skin show thickened basement membranes and reduced flow, impairing nutrient delivery and waste removal. Immunologically, skin becomes less responsive to injury and infection, increasing the risk of delayed healing and carcinogenesis.
Comparison: Intrinsic vs. Photoaging
| Feature | Intrinsic Aging | Photoaging |
| Epidermis | Thins over time | Stratum corneum thickens |
| Collagen | Gradual reduction | Accelerated degradation via MMPs |
| Elastic Fibers | Less defined | Replaced by clumped, non-functional material |
| Inflammation | Minimal | Persistent, UV-induced |
| Barrier Integrity | Mostly intact | Often impaired |
What These Mechanisms Mean for Skincare
If we want to influence how the skin ages, products must be designed to match the biology. That means not just selecting active ingredients, but understanding how they interact with these molecular pathways.
Key Targets for Formulation
- Oxidative damage: Ascorbyl tetraisopalmitate, ferulic acid, or other photostable antioxidants
- Collagen degradation: Retinoids to inhibit MMPs and stimulate synthesis
- Barrier repair: Ceramides, cholesterol, and free fatty acids in optimal ratios
- Cellular metabolism: Niacinamide for mitochondrial and barrier support
- DNA support (emerging area): Liposomal delivery of DNA-repair enzymes
Formulation Challenges
Active ingredients need to remain stable in the final formula and on the skin. This requires attention to pH, solubility, and oxidative degradation. Certain combinations—like unbuffered acids with retinoids or vitamin C with copper peptides—may destabilize each other or increase irritation.
Important Note: Just because an ingredient shows promise in vitro doesn’t mean it will perform the same way on real skin. Formulation science bridges that gap.
Final Thoughts: Aging Is a Biological Conversation, Not a Countdown
Skin ageing isn’t fixed in place. It shifts in response to your environment, your health, and how you care for it. When you understand the mechanisms at play, you’re not aiming for flawlessness—you’re choosing to care for your skin with insight, not guesswork.
Have you noticed changes in how your skin behaves? Are there ingredients or exposures you suspect may be influencing the process? Let’s break it down together.
Talk to you soon!
Dr Bozica
References:
https://www.sciencedirect.com/science/article/pii/S0965206X16000280
https://nyaspubs.onlinelibrary.wiley.com/doi/full/10.1196/annals.1404.027
https://www.nature.com/articles/s41598-021-01573-z
https://www.mdpi.com/1422-0067/22/23/12641
https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2023.1195272/full

